Amine gas purification from hydrogen sulfide: principle, effective options and installation schemes
The natural gas produced from the fields for delivery to the consumer through the pipelines contains sulfur compounds in different proportions. If they are not eliminated, aggressive substances will destroy the pipeline and render fittings unusable. In addition, toxins are released during the combustion of polluted blue fuel.
In order to avoid negative consequences, an amine gas purification from hydrogen sulfide is performed. This is the easiest and cheapest way to separate harmful components from fossil fuels. We will tell you how the process of separation of sulfur inclusions occurs, how the treatment plant is arranged and works.
The content of the article:
Purpose of fossil fuel treatment
Gas is the most popular type of fuel. It attracts the most affordable price and causing the least damage to the environment. Indisputable advantages include the simplicity of controlling the combustion process and the ability to secure all stages of fuel processing in the process of obtaining thermal energy.
However, natural gaseous fossil is not extracted in its pure form, because simultaneously with the extraction of gas from the well, associated organic compounds are pumped out. The most common of these is hydrogen sulfide, the content of which varies from tenths to ten or more percent, depending on the field.
Hydrogen sulfide is toxic, harmful to the environment, harmful to catalysts used in gas processing. As we have already noted, this organic compound is extremely aggressive with respect to steel pipes and metal valves.
Naturally, corroding the private system and gas main, hydrogen sulfide leads to leaks of blue fuel and the extremely negative, risky situations associated with this fact. To protect the consumer, unhealthy compounds are removed from the composition of the gaseous fuel before it is delivered to the highway.
According to the standards of hydrogen sulfide compounds, the gas transported through pipes cannot exceed 0.02 g / m³. However, in fact there are much more. In order to achieve the value regulated by GOST 5542-2014, cleaning is required.
Existing methods for separating hydrogen sulfide
In addition to hydrogen sulfide prevailing against other impurities, other harmful compounds may also be contained in blue fuel. You can find in it carbon dioxide, light mercaptans and carbon sulfide. But hydrogen sulfide itself will always prevail.
It is worth noting that some insignificant content of sulfur compounds in purified gaseous fuel is acceptable. The specific tolerance figure depends on the purpose for which the gas is produced. For example, for the production of ethylene oxide, the total sulfur content should be less than 0.0001 mg / m³.
The method of cleaning is chosen, focusing on the desired result.
All existing methods are divided into two groups:
- Sorption. They consist in the absorption of hydrogen sulfide compounds by a solid (adsorption) or liquid (absorption) reagent with the subsequent release of sulfur or its derivatives. After that, harmful impurities extracted from the gas composition are disposed of or recycled.
- Catalytic. They consist in the oxidation or reduction of hydrogen sulfide with its conversion to elemental sulfur.The process is implemented in the presence of catalysts - substances that stimulate the course of a chemical reaction.
Adsorption involves the collection of hydrogen sulfide by concentrating it on the surface of a solid. Most often, granular materials based on activated carbon or iron oxide are involved in the adsorption process. The large specific surface characteristic of grains contributes to the maximum retention of sulfur molecules.
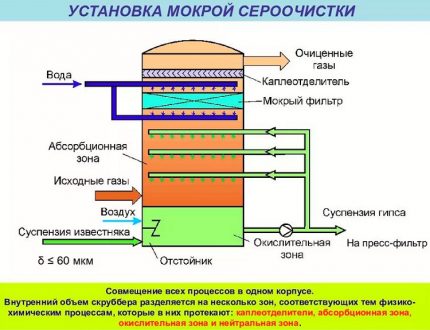
The absorption technology is characterized in that gaseous hydrogen sulfide impurities are dissolved in the active liquid substance. As a result, gaseous contaminants pass into the liquid phase. Then the selected harmful components are removed by evaporation, otherwise desorption, by this method they are removed from the reactive liquid.
Despite the fact that adsorption technology belongs to the “dry processes” and allows fine purification of blue fuel, absorption is most often used to remove contaminants from natural gas. The collection and elimination of hydrogen sulfide compounds using liquid absorbers is more profitable and appropriate.

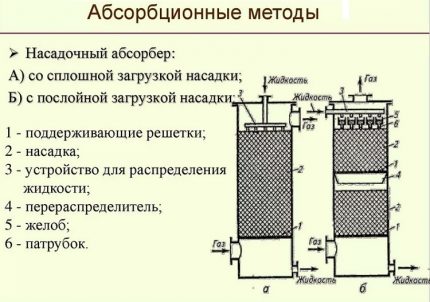
The absorption methods used in gas purification are divided into the following three groups:
- Chemical. Produced using solvents that freely react with hydrogen sulfide acidic pollutants. Ethanolamines or alkanolamines have the highest absorption capacity among chemical sorbents.
- Physical. Performed by physical dissolution of gaseous hydrogen sulfide in a liquid absorber. Moreover, the higher the partial pressure of the gaseous pollutant, the faster the dissolution process. Methanol, propylene carbonate, etc. are used here as an absorber.
- Combined. In the mixed version of the extraction of hydrogen sulfide, both technologies are involved. The main work is carried out by absorption, and fine tertiary treatment is carried out by adsorbents.
For half a century, the most popular and popular technology for the extraction and removal of hydrogen sulfide and carbonic acid from natural fuels has been the chemical purification of gas using an amine sorbent used in the form of an aqueous solution.
Amine technology is more suitable for processing large volumes of gas, because:
- Lack of deficit. Reagents can always be purchased in the volume required for cleaning.
- Acceptable Absorption. Amines are characterized by high absorption capacity. Of all the substances used, only they are able to remove 99.9% of hydrogen sulfide from gas.
- Priority characteristics. Aqueous amine solutions are distinguished by the most acceptable viscosity, vapor density, thermal and chemical stability, low heat capacity. Their characteristics provide the best course of the absorption process.
- No toxicity of reactive substances. This is an important argument convincing to resort specifically to the amine method.
- Selectivity. Quality required for selective absorption. It provides the possibility of sequentially carrying out the necessary reactions in the order required for an optimal result.
Ethanolamines used in carrying out chemical methods for cleaning gas from hydrogen sulfide and carbon dioxide include monoethanolamines (MEA), diethanolamines (DEA), triethanolamines (TEA). Moreover, substances with prefixes mono- and di- are eliminated from gas and H2S, and CO2. But the third option helps to remove only hydrogen sulfide.
When performing selective cleaning of blue fuel, methyldiethanolamines (MDEA), diglycolamines (DHA), and diisopropanolamines (DIPA) are used. Selective absorbents are mainly used abroad.
Naturally, ideal absorbents that meet all cleaning requirements before being delivered to the system. gas heating and supply of other equipment does not yet exist. Each solvent has some advantages along with minuses. When choosing a reactive substance, they simply determine the most suitable of the series proposed.
Typical installation principle
Maximum absorbency with respect to H2S is characterized by a solution of monoethanolamine. However, this reagent has a couple of significant disadvantages. It is characterized by rather high pressure and the ability to create irreversible compounds with carbon monoxide during the operation of the amine gas purification unit.
The first minus is eliminated by washing, as a result of which the amine vapor is partially absorbed. The second is rare in the processing of field gases.
The concentration of an aqueous solution of monoethanolamine is selected empirically, on the basis of the studies carried out it is taken to purify gas from a specific field. The selection of the percentage of reagent takes into account its ability to withstand the aggressive effects of hydrogen sulfide on the metal components of the system.
The standard absorbent content is usually in the range of 15 to 20%. However, it often happens that the concentration is increased to 30% or reduced to 10%, depending on how high the degree of purification should be. Those. for what purpose, in heating or in the production of polymer compounds, gas will be used.
Note that with an increase in the concentration of amine compounds, the corrosivity of hydrogen sulfide decreases. But we must take into account that in this case the reagent consumption increases. Consequently, the cost of purified commercial gas rises.
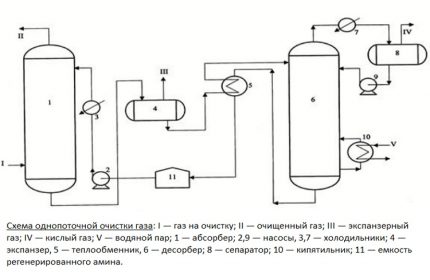
The main unit of the treatment plant is an absorber of a plate or mounted variety. This is a vertically oriented, externally resembling a test tube, apparatus with nozzles or plates located inside. In its lower part there is an entrance for the supply of crude gas mixture, at the top there is an exit to the scrubber.
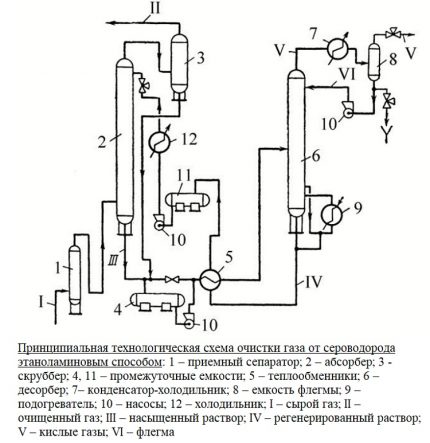
The gas flow after passing through the inlet separator is pumped into the lower section of the absorber. Then it passes through plates or nozzles located in the middle of the casing, on which contaminants settle. The nozzles, completely moistened with an amine solution, are separated by gratings for uniform distribution of the reagent.
Further, the blue fuel cleaned of impurities is sent to the scrubber. This device can be connected in the processing circuit after the absorber or located in its upper part.
The spent solution flows down the walls of the absorber and is sent to the distillation column - a stripper with a boiler. There, the solution is purified from the absorbed contaminants by the vapors released by boiling water in order to return back to the installation.
Regenerated, i.e. rid of hydrogen sulfide compounds, the solution flows into the heat exchanger. In it, the liquid is cooled during the transfer of heat to the next portion of the contaminated solution, after which it is pumped into the refrigerator by the pump for complete cooling and condensation of steam.
The cooled absorbent solution is again fed into the absorber. So the reagent circulates through the installation. Its vapors are also cooled and purified from acidic impurities, after which they replenish the supply of reagent.
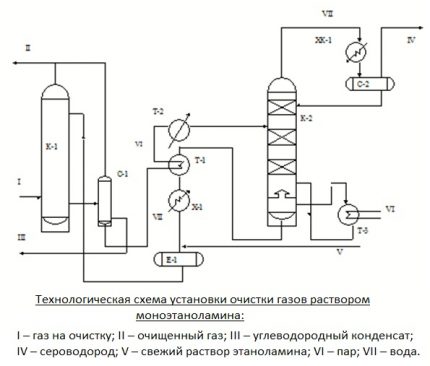
If it is necessary to carry out simultaneous removal of CO from the processed gas2 and H2S, two-stage cleaning is performed. It consists in the use of two solutions that differ in concentration. This option is more economical than single-stage cleaning.
First, gaseous fuel is cleaned with a strong composition with a reagent content of 25-35%. Then the gas is treated with a weak aqueous solution, in which the active substance is only 5-12%. As a result, both coarse and fine cleaning is carried out with a minimum flow rate of the solution and the rational use of the generated heat.
Four alkonolamine treatment options
Alkanolamines or amino alcohols are substances containing not only an amine group, but also a hydroxy group.
The apparatus and technology for purifying natural gas with alkanolamines differ mainly in the method of supplying an absorbent substance. Most often, four basic methods are used in gas purification using this type of amine.
First way. It determines the flow of the active solution in one stream from above. The entire amount of absorbent is sent to the upper plate of the installation. The cleaning process occurs at a temperature background of no higher than 40ºС.
This technique is usually used for minor contamination with hydrogen sulfide compounds and carbon dioxide. The total thermal effect for the production of commercial gas in this case, as a rule, is low.
Second way. This cleaning option is used for high levels of hydrogen sulfide compounds in gaseous fuels.
The reactive solution in this case is fed in two streams. The first, with a volume of about 65-75% of the total mass, is sent to the middle of the installation, the second is delivered from above.
The amine solution flows down the plates and meets upward gas streams that are pumped onto the bottom plate of the absorbent system. Before serving, the solution is heated to no more than 40 ° C, but during the interaction of the gas with the amine, the temperature rises significantly.
In order to prevent the cleaning efficiency from falling due to the temperature increase, excess heat is removed along with the spent solution saturated with hydrogen sulfide. And at the top of the installation, the stream is cooled in order to extract residual acidic components along with condensate.
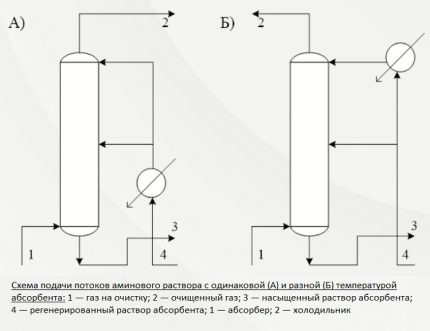
This is an economical way to reduce the consumption of both energy and active solution. Additional heating is not performed at any stage. In terms of technological essence, it is a two-level purification, which provides the opportunity with the least loss to prepare commercial gas for supply to the highway.
Third way. It assumes the supply of the absorber to the cleaning plant in two streams of different temperatures. The method is applied if, in addition to hydrogen sulfide and carbon dioxide, there is also CS in the raw gas2, and COS.
The predominant part of the absorber, approximately 70-75%, is heated to 60-70 ° C, and the remaining fraction only to 40 ° C. Flows are fed into the absorber in the same way as in the case described above: from above and in the middle.
The formation of a zone with a high temperature makes it possible to quickly and efficiently remove organic impurities from the gas mass at the bottom of the cleaning column. At the top, carbon dioxide and hydrogen sulfide are precipitated with an amine of standard temperature.
Fourth way. This technology determines the supply of an aqueous solution of amine in two streams with different degrees of regeneration. That is, one is supplied unrefined, containing hydrogen sulfide inclusions, the second without them.
The first stream cannot be called completely polluted. It only partially contains acidic components, because some of them are removed during cooling to + 50º / + 60ºC in the heat exchanger. This solution stream is taken from the lower nozzle of the stripper, cooled and sent to the middle part of the column.
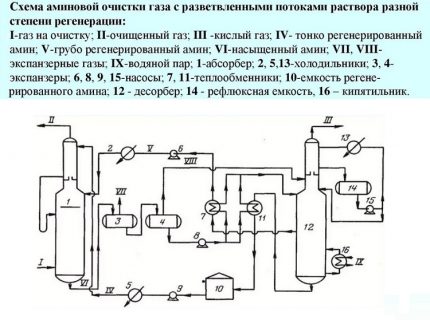
Only the part of the solution that is pumped into the upper sector of the installation passes through deep cleaning. The temperature of this stream usually does not exceed 50 ° C. Fine cleaning of gaseous fuels is carried out here. This design reduces costs by at least 10% by reducing steam consumption.
It is clear that the cleaning method is chosen based on the presence of organic pollutants and economic feasibility. In any case, a variety of technologies allows you to choose the best option. At the same amine gas treatment unit, the degree of purification can be varied, producing blue fuel with the right ones for work gas boilers, stoves, heaters characteristics.
Conclusions and useful video on the topic
The following video will familiarize you with the specifics of extracting hydrogen sulfide from associated gas extracted with oil from an oil well:
The installation of purification of blue fuel from hydrogen sulfide with the production of elemental sulfur for further processing will present the video:
The author of this video will tell you about how to get rid of biogas from hydrogen sulfide at home.
The choice of gas purification method is primarily oriented towards solving a specific problem. The artist has two ways: to follow a proven pattern or to prefer something new. However, the main guideline should still be economic feasibility while maintaining quality and obtaining the desired degree of processing.

 How to check for gas leaks at home: effective ways to check and deal with leaks
How to check for gas leaks at home: effective ways to check and deal with leaks  Battery gas leak sensors: operating principle and varieties + best brands on the market
Battery gas leak sensors: operating principle and varieties + best brands on the market  Where to call if there is no gas in the apartment: reasons for disconnection + procedure for the absence of gas
Where to call if there is no gas in the apartment: reasons for disconnection + procedure for the absence of gas  Types of domestic gas: what gas comes to our apartments + features of domestic gas
Types of domestic gas: what gas comes to our apartments + features of domestic gas  Natural gas odorant: features of odorants, norms and rules for their entry
Natural gas odorant: features of odorants, norms and rules for their entry  Actions for the smell of gas in the boiler room: what to do when a characteristic odor is detected
Actions for the smell of gas in the boiler room: what to do when a characteristic odor is detected  How much does it cost to connect gas to a private house: the price of organizing gas supply
How much does it cost to connect gas to a private house: the price of organizing gas supply  The best washing machines with dryer: model rating and customer tips
The best washing machines with dryer: model rating and customer tips  What is the color temperature of light and the nuances of choosing the temperature of the lamps to suit your needs
What is the color temperature of light and the nuances of choosing the temperature of the lamps to suit your needs  Replacement of a geyser in an apartment: replacement paperwork + basic norms and requirements
Replacement of a geyser in an apartment: replacement paperwork + basic norms and requirements